What Is The Atomic Number of Carbon 13: Essential Guide
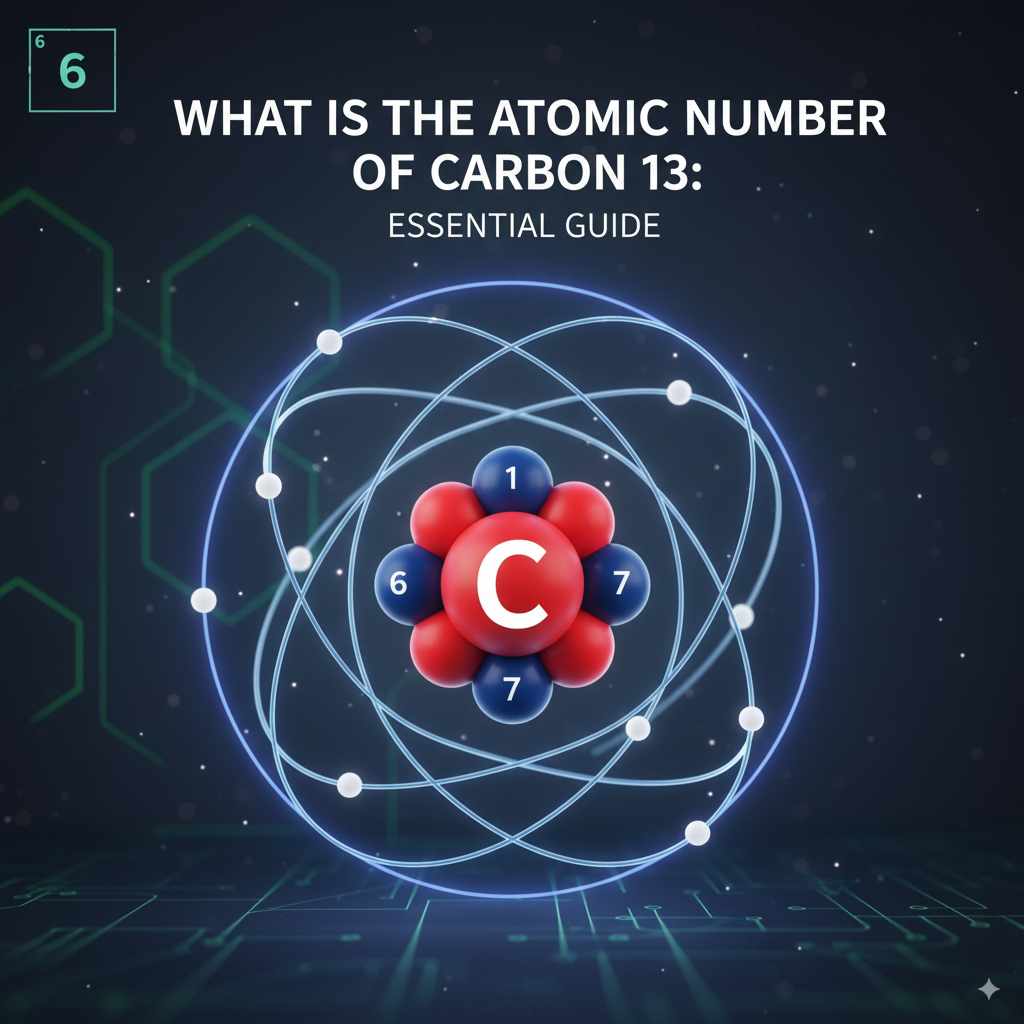
The atomic number of Carbon-13 is 6. This number refers to the protons in the atom’s nucleus, which defines it as carbon. Carbon-13 is an isotope of carbon, meaning it has a different number of neutrons than the more common carbon-12.
Hey there! Ever looked at a chemical element and wondered what all those numbers mean? You’re definitely not alone. Sometimes, science stuff can feel a bit like deciphering a secret code, especially when you start hearing terms like “Carbon-13” and “atomic number” thrown around. It can sound complicated, but trust me, it’s easier to understand than you might think. We’re going to break down exactly what the atomic number of Carbon-13 is and why it matters, in a way that makes perfect sense.
Think of it like this: just like every car has a unique Vehicle Identification Number (VIN) that tells you exactly what kind of car it is, every element has an “atomic number.” This number is super important because it’s the key to identifying an element. We’ll get into what makes Carbon-13 special and how its atomic number remains the same, even with a slightly different weight.
By the end of this guide, you’ll feel confident understanding what the atomic number of Carbon-13 is and grasp a bit more about the building blocks of everything around us. Ready to dive in and demystify this?
Understanding the Basics: Atoms, Elements, and Atomic Numbers
Before we zoom in on Carbon-13, let’s get a handle on the fundamental stuff: atoms, elements, and what an atomic number really is. Imagine everything you see – your car, the road, the air you breathe – is built from tiny, tiny LEGO bricks. These bricks are called atoms.
An element is like a specific type of LEGO brick. For example, you have red bricks, blue bricks, and yellow bricks. In the world of science, we have elements like Hydrogen, Oxygen, and Carbon. Each element is defined by the number of tiny particles inside its core, called protons.
The atomic number is simply the count of protons in an atom’s nucleus (the center of the atom). This number is the ultimate identifier for an element. If an atom has 6 protons, it’s always carbon. If it has 8 protons, it’s always oxygen. It’s like a fingerprint for each element!
Protons: The Key Identifiers
Protons are positively charged particles found in the nucleus of an atom. They’re like the element’s identity card. No matter what else changes with an atom of a specific element, the number of protons – and therefore the atomic number – stays the same. This is a fundamental rule in chemistry.
Neutrons and Electrons: Partners in the Atom
While protons are the defining feature, atoms also have other particles:
- Neutrons: These are neutral particles (no charge) also found in the nucleus. They add mass to the atom but don’t change which element it is.
- Electrons: These are tiny, negatively charged particles that orbit the nucleus. In a neutral atom, the number of electrons is equal to the number of protons, balancing out the charges.
What Makes Carbon-13 Unique? Isotopes Explained
Now, let’s talk about Carbon-13. You might have heard of carbon before – it’s the backbone of organic chemistry, found in everything from living things to fuel. Most of the carbon you encounter in everyday life is something called Carbon-12.
But just like you can have different models of the same car (like a sedan versus an SUV, both being “cars”), elements can have different “versions.” These versions are called isotopes.
Isotopes of an element have the same number of protons (that’s what makes them the same element), but they have a different number of neutrons. This difference in neutrons means isotopes of an element have different masses.
So, when we talk about Carbon-13, we’re talking about an isotope of carbon. It has the same number of protons as all other carbon atoms, but it has more neutrons.
Carbon-12 vs. Carbon-13
Let’s break down the two most common forms of carbon:
| Feature | Carbon-12 (12C) | Carbon-13 (13C) |
|---|---|---|
| Number of Protons | 6 | 6 |
| Number of Neutrons | 6 | 7 |
| Mass Number (Protons + Neutrons) | 12 | 13 |
| Abundance in Nature | About 98.9% | About 1.1% |
As you can see, both Carbon-12 and Carbon-13 have 6 protons. This is crucial because, as we learned, the number of protons defines the element. So, the atomic number for both Carbon-12 and Carbon-13 is 6.
The “12” and “13” in their names refer to their mass number, which is the total count of protons and neutrons in the nucleus. Carbon-12 has 6 protons + 6 neutrons = 12. Carbon-13 has 6 protons + 7 neutrons = 13. This extra neutron in Carbon-13 makes it slightly heavier than Carbon-12.
So, What Is The Atomic Number of Carbon 13?
To put it simply and directly, the atomic number of Carbon-13 is 6. This is because Carbon-13 has 6 protons in its nucleus. The atomic number is the fundamental characteristic that labels an element as carbon, regardless of how many neutrons it has.
The number “13” in Carbon-13 refers to its mass number, not its atomic number. It tells us that this specific type of carbon atom has a nucleus containing 6 protons and 7 neutrons.
Why is the Atomic Number So Important?
The atomic number is the key to the entire periodic table. It’s how all the elements are organized. Elements are arranged in order of increasing atomic number. This order reveals patterns in their chemical properties, which is why the periodic table is such a powerful tool for scientists.
- An atomic number of 1 means Hydrogen.
- An atomic number of 2 means Helium.
- An atomic number of 6 means Carbon.
- An atomic number of 8 means Oxygen.
This consistent numbering system allows scientists to predict how elements will behave and interact with each other. Even though Carbon-13 and Carbon-12 are isotopes with different masses, they behave very similarly chemically because they both have 6 protons and the same number of electrons (in a neutral atom).
Applications and Uses of Carbon-13
You might be wondering, “If Carbon-13 is just a heavier version of carbon, why do we even talk about it?” Well, this difference in mass and its unique properties make Carbon-13 incredibly useful in many scientific fields.
1. Medical Imaging and Diagnostics
Carbon-13 is a crucial component in medical technologies. For example, it’s used in tests to detect infections like Helicobacter pylori, a bacterium that can cause stomach ulcers. A patient might ingest a special drink containing a Carbon-13 labeled compound. The bacteria consume this compound, and a breath test can detect the Carbon-13 as it’s exhaled, indicating an infection. This is a non-radioactive and safe method.
You can find more details on these diagnostic uses from resources like the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK).
2. Scientific Research and Tracing
In scientific research, Carbon-13 is used as a “tracer.” Because it’s stable (not radioactive) and detectable, scientists can follow carbon atoms through complex biological, chemical, or environmental processes. For instance, in photosynthesis research, scientists can label carbon dioxide with Carbon-13 to track how plants use it to create sugars.
3. Nuclear Magnetic Resonance (NMR) Spectroscopy
Carbon-13 NMR is a powerful analytical technique used to determine the structure of organic molecules. While Carbon-12 is not easily detectable by NMR, Carbon-13 has a nuclear spin that makes it “visible” to NMR machines. This allows chemists to identify and analyze the carbon skeleton of a molecule with great detail.
For a deeper dive into NMR, reputable sources like LibreTexts Chemistry offer extensive explanations.
4. Radiometric Dating (Indirectly)
While Carbon-14 (a radioactive isotope) is used for dating very old organic materials, understanding the ratios of stable isotopes like Carbon-12 and Carbon-13 can also provide valuable environmental and climate information, especially when looking at historical carbon cycles.
Frequently Asked Questions (FAQs) About Carbon-13’s Atomic Number
Q1: What is the atomic number of Carbon-13?
The atomic number of Carbon-13 is 6. This is because it has 6 protons in its nucleus, which is the defining characteristic of all carbon atoms.
Q2: Does Carbon-13 have 13 protons then?
No, Carbon-13 does not have 13 protons. The “13” in Carbon-13 refers to its mass number, which is the total count of protons and neutrons in the nucleus. It has 6 protons and 7 neutrons.
Q3: What is the difference between Carbon-12 and Carbon-13?
The main difference is the number of neutrons. Carbon-12 has 6 neutrons, while Carbon-13 has 7 neutrons. This makes Carbon-13 slightly heavier than Carbon-12. Both have 6 protons and thus the same atomic number (6).
Q4: Are Carbon-13 atoms radioactive?
No, Carbon-13 is a stable isotope. This means it does not undergo radioactive decay. This stability is what makes it useful for tracing and analytical techniques where radioactivity would interfere.
Q5: Why is Carbon-13 important in medicine?
Carbon-13 is used in medical diagnostics, such as breath tests to detect certain infections like H. pylori. Since it’s not radioactive, it can be safely ingested by patients to track biological processes.
Q6: How is the atomic number determined?
The atomic number of an element is determined by counting the number of protons in the nucleus of an atom of that element. This count is unique to each element.
Q7: Can Carbon-13 be found naturally?
Yes, Carbon-13 occurs naturally on Earth. It makes up about 1.1% of all naturally occurring carbon, with Carbon-12 being far more common at about 98.9%.
Conclusion: The Enduring Identity of Carbon
So, there you have it! We’ve journeyed from the tiny building blocks of matter to the specific identity of Carbon-13. The core takeaway is clear: the atomic number of Carbon-13 is, and always will be, 6. This number isn’t just a random digit; it’s the fundamental identifier that places it firmly in the carbon family, no matter how many neutrons it carries.
Understanding atomic numbers and isotopes like Carbon-13 helps us appreciate the incredible diversity and complexity within the seemingly simple world of elements. From powering life as we know it to enabling cutting-edge medical tests and scientific analysis, carbon, in all its isotopic forms, plays a vital role. You’ve successfully demystified a key concept in chemistry, proving that even the most technical topics can be grasped with clear explanations.
Keep exploring, stay curious, and remember that mastering these basics can open up a whole new understanding of the world around you. Happy learning!