What Is The Atomic Number of Carbon 13: Essential Facts
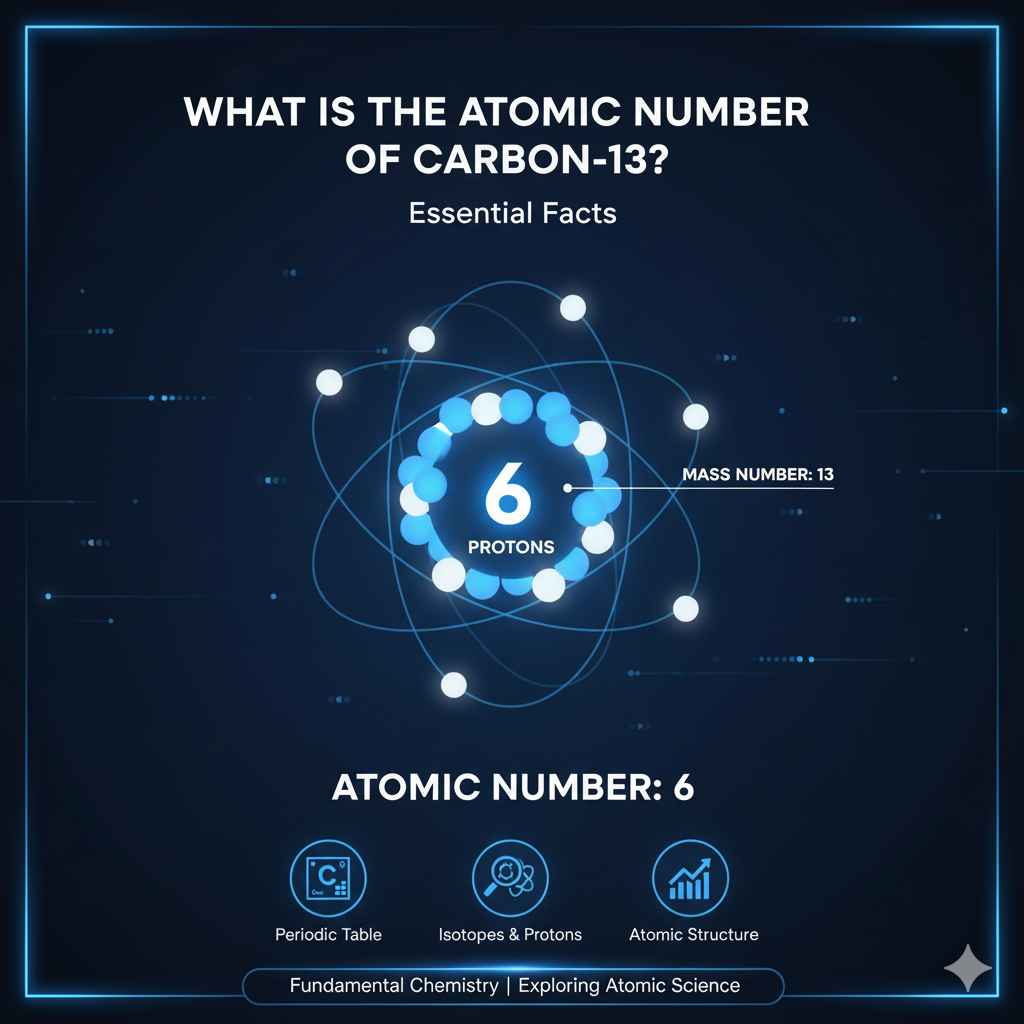
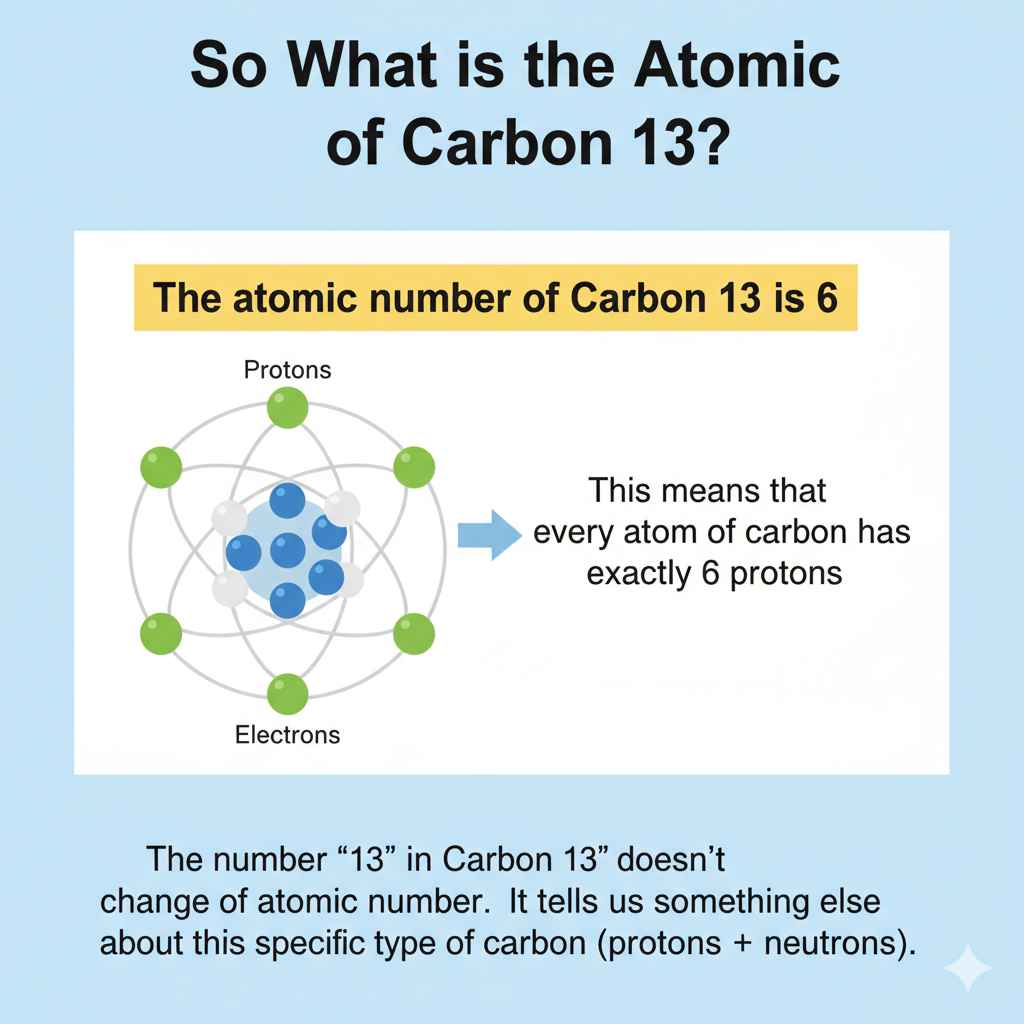
The atomic number of Carbon 13 is 6. This fundamental number tells us carbon always has 6 protons, defining it as the element carbon. Carbon 13 is a specific type, or isotope, of carbon.
Understanding Carbon 13: What’s Its Atomic Number?
Ever wondered about the building blocks of everything around us? We see cars, roads, and even ourselves made of tiny particles. At the heart of these particles are atoms, and each type of atom has a unique identifier. This is where the atomic number comes in. If you’ve heard about Carbon 13 and are curious about its atomic number, you’re in the right place. It’s simpler than it sounds, and understanding it helps us grasp the amazing world of chemistry. We’ll break it all down in easy steps, so by the end, you’ll feel confident about what Carbon 13 is and why its atomic number matters.
What is an Atomic Number?
Think of the atomic number as an atom’s unique ID card. It’s the number of protons found in the nucleus (the very center) of an atom. Protons are positively charged particles. Every atom of a specific element always has the same number of protons. For instance, all hydrogen atoms have 1 proton, all helium atoms have 2 protons, and so on. This number is what defines an element.
So, What is the Atomic Number of Carbon 13?
Here’s the most important thing to remember: The atomic number of Carbon 13 is 6.
This means that every atom of carbon, no matter what type it is, has exactly 6 protons. The number ’13’ in Carbon 13 doesn’t change the atomic number. It tells us something else about this specific type of carbon.
The Difference: What Makes Carbon 13 Special?
If the atomic number is always 6 for carbon, what does the ’13’ mean? That number refers to the mass number of that specific carbon atom.
Atomic Number: Number of protons (always 6 for carbon).
Mass Number: The total number of protons and neutrons in an atom’s nucleus.
So, Carbon 13 has 6 protons and a total of 13 protons plus neutrons. To figure out the number of neutrons, we do a simple calculation:
Mass Number – Atomic Number = Number of Neutrons
13 – 6 = 7 neutrons
This means Carbon 13 has 6 protons and 7 neutrons in its nucleus.
Exploring Isotopes: Carbon’s Family
Atoms of the same element that have different numbers of neutrons are called isotopes. They are like siblings in a family – they share the same core identity (proton number) but have slight differences (neutron number).
Carbon has several isotopes, but the most common ones are Carbon 12 and Carbon 13.
Carbon 12 (¹²C)
Protons: 6
Neutrons: 6 (Mass Number 12 = 6 protons + 6 neutrons)
This is the most abundant form of carbon on Earth, making up about 99% of all carbon.
Carbon 13 (¹³C)
Protons: 6
Neutrons: 7 (Mass Number 13 = 6 protons + 7 neutrons)
This isotope is less common, making up about 1% of all carbon.
There’s also Carbon 14 (¹⁴C), which is famous for its use in radiocarbon dating. It has 6 protons and 8 neutrons. Unlike Carbon 12 and 13, Carbon 14 is radioactive.
Why Does the Atomic Number Matter?
The atomic number is super important because it dictates everything about an element:
1. Identity: It’s what makes an element what it is. An atom with 6 protons will always be carbon, regardless of its neutrons or electrons.
2. Chemical Behavior: The number of protons influences the number of electrons an atom has (in a neutral atom, protons = electrons). Electrons are key players in how atoms bond with each other to form molecules. So, the atomic number indirectly determines an element’s chemical properties.
3. Position on the Periodic Table: Elements are arranged on the periodic table according to their atomic numbers. This organized system helps scientists predict and understand the relationships between different elements. You can see carbon clearly listed with its atomic number 6.
Essential Facts About Carbon 13
Let’s sum up some key facts about Carbon 13 (¹³C), focusing on its atomic number and what makes it distinct.
Atomic Number: 6 (meaning it has 6 protons).
Mass Number: 13 (meaning it has 6 protons + 7 neutrons).
Stability: Carbon 13 is a stable isotope. It does not decay radioactively like Carbon 14. This stability is a key difference.
Occurrence: It’s a naturally occurring isotope of carbon, found in small amounts alongside the more common Carbon 12.
Significance: While it’s not as abundant as Carbon 12, it plays important roles in scientific research and analysis.
Table: Comparing Carbon Isotopes
Here’s a simple table that helps visualize the difference between the main carbon isotopes:
| Feature | Carbon 12 (¹²C) | Carbon 13 (¹³C) |
|---|---|---|
| Atomic Number | 6 | 6 |
| Number of Protons | 6 | 6 |
| Number of Neutrons | 6 | 7 |
| Mass Number | 12 | 13 |
| Stability | Stable | Stable |
| Abundance on Earth | ~99% | ~1% |
How is Carbon 13 Used?
Even though Carbon 13 is less common than Carbon 12, it’s incredibly useful in science and medicine. Its natural stability makes it a great tool.
1. Medical Diagnostics
Carbon 13 is used in breath tests to detect certain medical conditions like infections caused by Helicobacter pylori (a common cause of stomach ulcers). The patient drinks a special liquid containing a molecule tagged with a non-radioactive Carbon 13. If the bacteria are present, they break down the molecule, releasing labeled carbon dioxide that the patient exhales. Doctors can then measure the Carbon 13 in the breath to diagnose the infection. This is a safe and non-invasive method. You can learn more about these diagnostic techniques through resources like the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK).
2. Scientific Research
NMR Spectroscopy: Carbon 13 has a property called “spin,” which makes it detectable by Nuclear Magnetic Resonance (NMR) spectroscopy. This technique is vital in chemistry for identifying the structure of molecules. By studying how Carbon 13 atoms behave in a magnetic field, scientists can understand complex chemical compositions.
Tracing Biochemical Pathways: Researchers use Carbon 13 as a “tracer” to follow molecules through biological processes in plants, animals, and humans. Since it’s stable and can be distinguished from Carbon 12, scientists can track carbon atoms as they move through metabolic pathways without introducing radioactivity. This helps us understand how living things use energy and build tissues.
Paleoclimate Studies: Analyzing the ratio of Carbon 13 to Carbon 12 (¹³C/¹²C ratio) in ancient materials like tree rings, ice cores, and fossilized shells can provide clues about past climate conditions and the carbon cycle. This is because plants and oceans preferentially absorb certain isotopes of carbon.
3. Material Science
In material science, Carbon 13 can be used to study the structure and properties of new materials, especially those involving carbon, like advanced polymers or composites.

Frequently Asked Questions (FAQ)
Here are some common questions beginner learners have about the atomic number of Carbon 13.
Q1: What is the atomic number of carbon, in general?
The atomic number of any carbon atom is always 6. This is because all carbon atoms have 6 protons in their nucleus. The atomic number identifies the element itself.
Q2: Is Carbon 13 radioactive?
No, Carbon 13 is a stable isotope. It does not undergo radioactive decay. This is different from Carbon 14, which is radioactive and used for dating. The stability of Carbon 13 makes it useful for medical tests and chemical analysis.
Q3: How is Carbon 13 different from Carbon 12?
Carbon 13 and Carbon 12 are both types of carbon (isotopes). They both have 6 protons, which is why they are both carbon. The difference is in the number of neutrons. Carbon 12 has 6 neutrons, and Carbon 13 has 7 neutrons. This gives Carbon 13 a slightly higher mass.
Q4: What does the ’13’ in Carbon 13 actually mean?
The ’13’ in Carbon 13 refers to its mass number. The mass number is the total count of protons and neutrons in the atom’s nucleus. So, Carbon 13 has 6 protons and 7 neutrons (6 + 7 = 13).
Q5: If Carbon 13 has 6 protons, why isn’t it called element number 6?
It is element number 6! The atomic number, which is the number of protons, is what defines an element. Since Carbon 13 has 6 protons, its atomic number is 6, identifying it as carbon. The ’13’ is the mass number, which describes a specific isotope of carbon.
Q6: Where can I find the atomic number of carbon on a periodic table?
You can easily find the atomic number on any standard periodic table. Carbon (symbol C) is usually located in Group 14. Its atomic number, 6, will be displayed prominently, often above the element symbol.
Understanding Atomic Mass vs. Atomic Number
It’s really important to distinguish between atomic number and atomic mass.
Atomic Number: Always tells you the number of protons. For Carbon, it’s always 6.
Atomic Mass: This is more complex. The atomic mass you see on the periodic table (for carbon, it’s about 12.01) is an average of the masses of all naturally occurring isotopes of that element, weighted by their abundance. So, it’s close to the mass of Carbon 12 because Carbon 12 is so common.
Mass Number: This is the exact count of protons and neutrons for a specific* isotope, like the 13 in Carbon 13.
Conclusion: A Solid Understanding of Carbon’s Identity
We’ve explored the world of carbon, focusing on its fundamental building block: the atomic number. You now know that the atomic number of Carbon 13 is, like all carbon atoms, 6. This number, representing the 6 protons in its nucleus, is the key identifier for the element carbon.
The ’13’ in Carbon 13 tells us it’s a specific isotope with 7 neutrons, making its mass number 13. This stable isotope, while less common than Carbon 12, plays a vital role in everything from precise medical diagnostics to deep scientific research. Understanding atomic numbers and isotopes helps us appreciate the incredible diversity and complexity of the elements that make up our universe. So, the next time you hear about Carbon 13, you’ll know exactly what its atomic number signifies and why it’s a unique and valuable form of carbon.